· customer story · 4 min read
R4J and T4J: Simple Solutions for Complex Medical Technology Regulations

Are you looking for tool support for your requirements and test management flow? Learn more about the problems that CogniMed was trying to solve and how Ease Solutions was able to help them.
With the combination of R4J and T4J, we have not only found very good and easy-to-use tools for our requirements, they can also be used economically in our company.
Prof. Dr. Petra Margaritoff, CogniMed GmbH
CogniMed, a specialist for technical development services in regulated industries such as medical technology and aviation, was founded in 1994 as a company for medical and industrial measurement and automation technology. CogniMed develops hardware, software and complete products for customers in these regulated industries. CogniMed’s specialty lies in perfectly documented development services for this technically demanding environment. On request CogniMed also produces prototypes, pre-series and small series. For one of its customers CogniMed has been manufacturing control and alarm components for the gas supply system in hospitals for over 15 years. In addition to product development of the electronics, CogniMed also designs and manufactures the service and production tools.
The Challenge
In medical technology or aviation, it is essential to comply with given standards and regulations under all circumstances. Standardards-compliant development documentation is crucial, which is why CogniMed depends on being able to bidirectionally trace products to their development sources at all times. This could theoretically be done with classic documentation tools such as spreadsheets, but the complexity makes this inefficient and unsuitable in practice. For a small specialist company like CogniMed, the standard tools for requirements documentation commonly used in the industry were economically out of the question, due to time-consuming administration and high acquisition costs.
The Solution
The company was looking for a cost-efficient tool with a convincing total cost of ownership, low integration effort, easy handling and administration. The desired solution should make a valuable contribution to meeting the stringent requirements of the demanding industries in which CogniMed operates. When deciding on a suitable tool, a number of functionalities were indispensable for CogniMed:
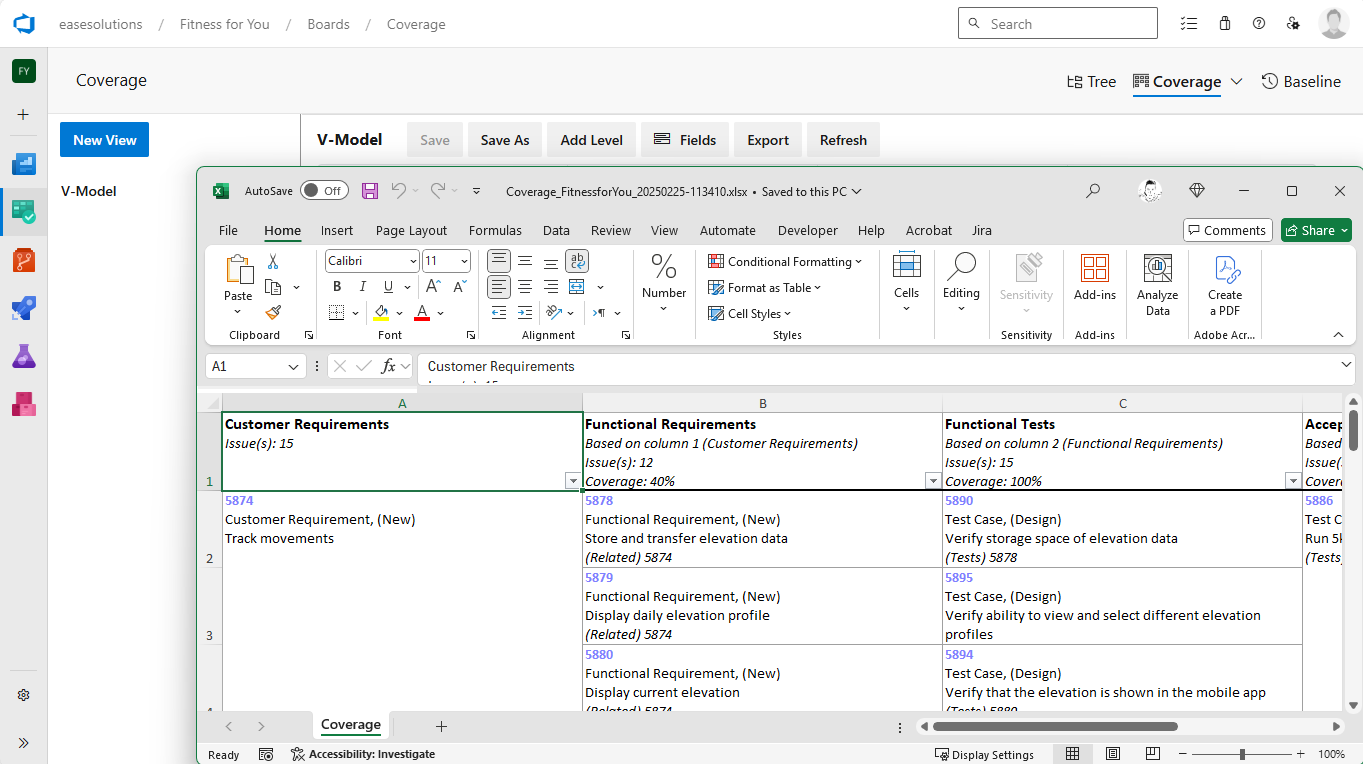
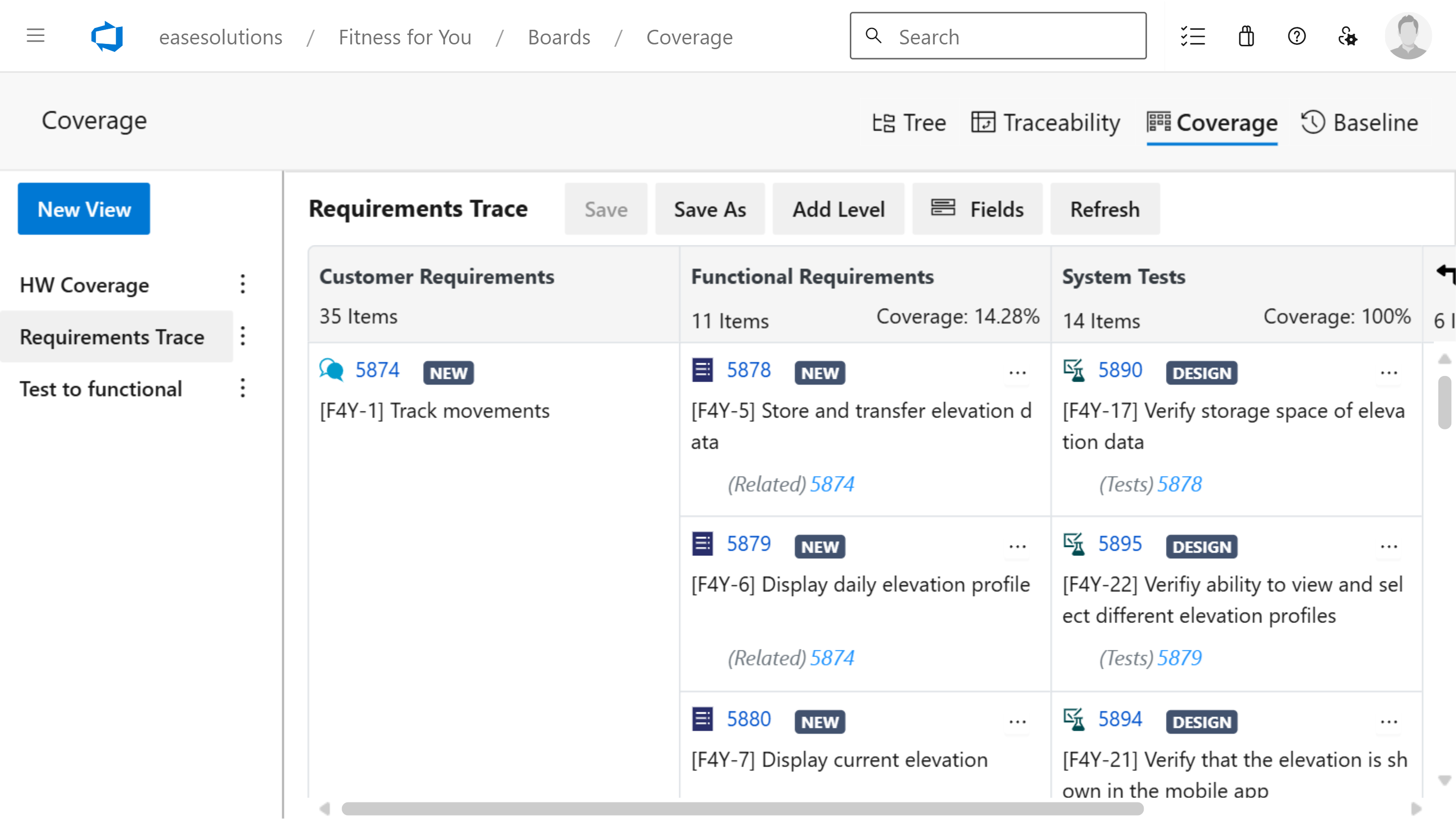
- The possibility of tracing development results over several levels
- Determing the coverage over these levels
- Exporting requirements from the system in a structured manner and in a defined order as a formatted text document
- The ability to distinguish between product, service and manufacturing tool requirements
- Support for including graphics in requirements
- Tracking authorship and changes with respect to baselines
“Finding a suitable tool, for the first point in particular, has proven to be unexpectedly difficult” says Petra Margaritoff of CogniMed. As a development company, CogniMed has been using Atlassian™ Jira® for years for all tracking needs. So it made sense to look into the Atlassian Marketplace as well where CogniMed found two apps provided by Ease Solutions that meet their demanding vision and are cost-effective at the same time.
R4J - Requirements Management for Jira ensures standards compliance throughout the development lifecycle. It is complemented by T4J - Test Management for Jira to effortlessly create test cases customized to the product and to document and track the results for each test step.
Conclusion
Due to their extensive functionality, optimal Jira integration and unbeatable price-performance ratio, R4J and T4J fully meet CogniMed’s requirements. Ease Solutions has proven to be a reliable partner for CogniMed over the past years, always offering a quick solution when problems arise and always demonstrating their ability to innovate.
We have been using R4J and T4J for many years now and are very satisfied. We can say that this is exactly the right choice of tool for our needs. Customers have themselves acquired a Jira instance with R4J and T4J after learning how to work with it at our company. This was the case for a customer, whose existing software we documented and updated for him. He is still happy to have found such flexible and custom-fit tools with Jira and the AddOn.
Frank Willers, CogniMed GmbH
Did you know that you can try out our products free of charge?
Sign up for the next demo session now to learn more about our products.